Business
Business
KinoPharma is developing a novel antiviral drug to treat HPV infections that cause cervical cancer.
Each year, 570,000 women develop cervical cancer, and more than half of that, 310,000, die. It has been elucidated that 99% of the causes of cervical cancer are persistent human papillomavirus (HPV) infections of the cervix.
HPV infection of the cervix is a sexually transmitted disease. Infected HPV may be eliminated by hosts’ immune system, but some infections become persistent. Persistent HPV infection develops into cervical intraepithelial neoplasia (CIN, also called cervical dysplasia), which is a precancerous disease of cervical cancer, and the dysplasia gradually progresses from mild grade called CIN1, to CIN2 as moderate grade, through CIN3 as severe grade, and finally becomes cancerous.
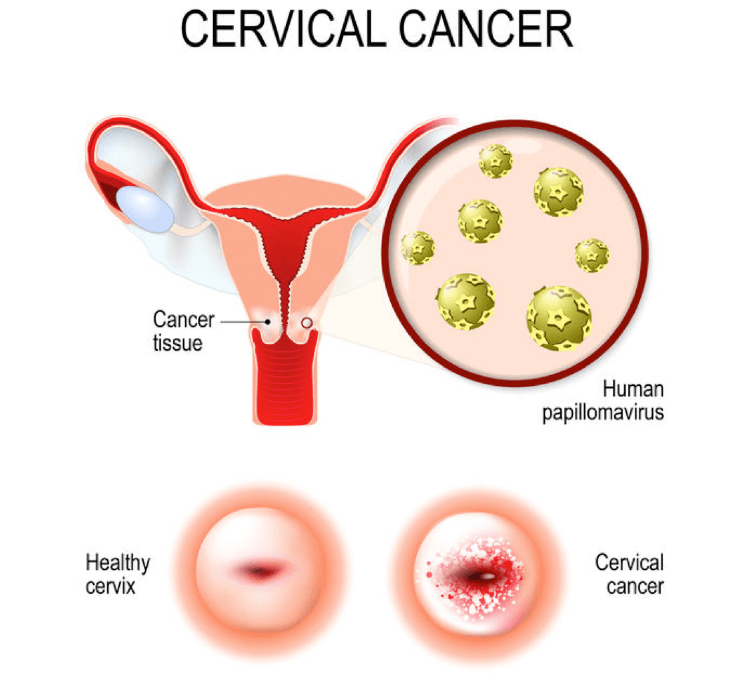
HPV infection of the cervix is a sexually transmitted disease. Infected HPV may be eliminated by hosts’ immune system, but some infections become persistent. Persistent HPV infection develops into cervical intraepithelial neoplasia (CIN, also called cervical dysplasia), which is a precancerous disease of cervical cancer, and the dysplasia gradually progresses from mild grade called CIN1, to CIN2 as moderate grade, through CIN3 as severe grade, and finally becomes cancerous.
It is envisioned that effective removal of HPV from cervical tissue in CIN patients before it becomes malignant will result in recovery and normalization of the tissue. Unfortunately, there are no approved antiviral drugs that can effectively eliminate HPV in CIN patients.
The HPV vaccine was developed to prevent HPV infection and is already approved in many countries. There is medical data that the incidence of cervical cancer can be suppressed by 70% with HPV vaccine. However, women who are unable to receive vaccination before their sexual experiences would not fully benefit from this. Unfortunately, with the exception of a few European countries and Australia, HPV vaccination coverage remains low in many other countries.
For patients who already have the severe degree CIN, surgery is available to remove the cervix in a conical shape before it becomes cancerous. This surgical measure manages to save the uterus itself and opens the way to future pregnancies, but medical experts point out that it may increase the risk of preterm birth. At the same time, many patients are unaware of the progression of CIN, miss the opportunity for conization and develop cervical cancer.
Applying our novel antiviral agents, KinoPharma is working on developing an effective drug for CIN which currently has no treatment other than cervical resection. Clinical trials have already begun in multiple countries, and we are aiming to obtain approval as soon as possible.
KinoPharma discovers, researches and develops first-in-class small molecule drugs for the disorders with unmet medical needs in optimal administration required for the disease, including systemic and local administration.
We target attractive and unexplored market areas by providing first-in-class drugs. At the same time, taking the advantage of small molecule drugs and also taking into account scalable marketability, we aim to provide drugs into emerging and developing countries where medical demand is expected to increase in the shortest future at a cost that can be shared with patients in need.
We have established a system of research and development which combines the wisdom of the academia and the speed of a biotech company, including the clinical studies led by medical doctors from Kyoto University based on the joint development agreement.
Our Kyoto Research Institute is located in the "Kyoto University Katsura Venture Plaza," a new industrial base for industry-academia-government collaboration located adjacent to the Katsura Campus of Kyoto University, and will promote research and development through close collaboration with Kyoto Unibersity.